Elon Musk’s Neuralink has secured a pivotal $43 million in funding, spearheaded by Peter Thiel’s Founders Fund. The latest injection propels it’s total funding to an impressive $323 million, setting the stage for imminent human trials. The show involves the development of a revolutionary brain implant device designed to monitor brain activity, gaining FDA approval in May. As for who, the venture is led by visionary entrepreneur Elon Musk. This significant financial boost comes amidst controversies surrounding monkey testing and increased regulatory scrutiny, shaping the narrative of Neuralink’s journey.
Contents
Advancing Towards Human Trials
Neuralink, Musk’s groundbreaking brain implant venture, aims to commence human trials. The company, armed with FDA approval obtained in May, is set to test its revolutionary implant on 11 individuals next year. The microchip, designed for insertion into the brain through the skull, will monitor brain activity.
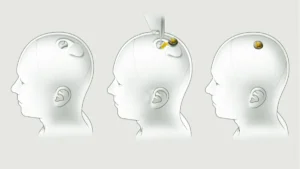
Controversies Surrounding Neuralink
Despite these advancements, Neuralink faces mounting scrutiny, particularly regarding its treatment of test subjects. Recent criticism has centered around the deaths of monkeys used in chip testing. An animal-rights group, the Physicians Committee for Responsible Medicine, alleged mistreatment, presenting records indicating “extreme suffering” and health issues leading to euthanasia.
Neuralink Controversies
| Controversy | Response from Neuralink |
|---|---|
| Monkey Testing Deaths | Elon Musk denies fatalities, cites use of terminal monkeys |
| Internal Challenges and Culture | Neuralink faces allegations of a “culture of blame and fear” |
| Regulatory Scrutiny and SEC Investigation | SEC urged to investigate Musk for securities fraud |
Check out our latest video to learn more about our PRIME Study! 🧠📱 pic.twitter.com/7zTMFzdZsF
— Neuralink (@neuralink) November 22, 2023
Response to Criticism and Regulatory Scrutiny
Elon Musk, responding to the allegations, asserted in September that “no monkey has died as a result of Neuralink’s chips.” He explained the use of terminal monkeys for early implants, minimizing risks to healthy subjects. However, four House representatives called for an SEC probe into Musk for securities fraud related to statements on the implants.
Must Read – The Rise of On-Device AI: Transforming the Tech Landscape
Internal Challenges at Neuralink
Internal challenges at Neuralink have also come to light. Former employees described a “culture of blame and fear,” prompting the departure of founding scientists. Anonymous sources revealed concerns about the company’s practices, raising questions about its work environment and potential impact on innovation.
“Neuralink isn’t just about connecting minds; it’s about unlocking the infinite potential within each of us, rewriting the future of human cognition, and ensuring that our synergy with technology is a leap forward, not just an evolution.” – Elon Musk

Controversial SEC Investigation Requests
The Physicians Committee for Responsible Medicine urged the SEC to investigate Musk and Neuralink for securities fraud, alleging misleading statements about monkey experiments. Despite a USDA ruling in July finding “no evidence” of animal welfare violations, the committee criticized it as a “free pass” for Neuralink. Recently, four Democratic House representatives joined the call for an SEC investigation into the company.
As Neuralink secures substantial funding for its groundbreaking brain implant technology, it simultaneously grapples with controversies and regulatory challenges. The balance between innovation and ethical practices remains a focal point, with internal concerns and external scrutiny shaping the narrative around Musk’s ambitious venture. The journey towards human trials and the potential impact on AI development continue to captivate industry observers and investors alike.
FAQs
Q1: What is Neuralink’s latest funding round about?
A: Neuralink secures an additional $43 million, led by Peter Thiel’s Founders Fund, gearing up for upcoming human trials.
Q2: How much funding has Neuralink raised so far?
A: The recent $43 million injection brings Neuralink’s total funding to an impressive $323 million.
Q3: When is Neuralink planning to conduct human trials?
A: Human trials are slated for the upcoming year, following FDA approval obtained in May.
Q4: What controversies surround Neuralink’s monkey testing?
A: Allegations of mistreatment and euthanasia have emerged, with Elon Musk asserting no fatalities resulted from the chip implants.
Q5: Why is Neuralink facing SEC scrutiny?
A: Four House representatives call for an SEC probe into Musk for securities fraud over statements regarding monkey experiments.
